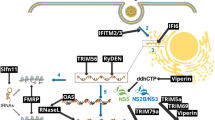
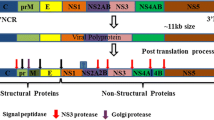
Abstract
Purpose of Review
The purpose of this review is to feature several cytokine families and describe the roles they play in mediating protection or pathogenesis at the different stages of flavivirus infections. We highlight the similarities and differences in the roles these cytokines play in the different flaviviruses.
Recent Findings
Cytokines play a role at every stage in flaviviral disease. In the past 3 years, exciting work has been done showing immunomodulatory effects of arthropod saliva on infection, novel roles of cytokines like IFNγ and TNFα in controlling acute flaviviral infection, critical roles of type III IFNs in limiting viral spread particularly in the neurotropic or teratogenic flaviviruses, and the detrimental effects of the inflammasome in recovery from neurotropic flavivirus infection.
Summary
Disease induced by flaviviral infection is the result of the complex, cytokine-driven interactions between host, virus, and vector and is distinct at each stage of infection. Though flaviviruses are similar in phylogeny, the mechanisms by which they cause disease can be distinct. However, a common theme persists, in that dysregulation of cytokines can cause devastating effects for the host. A thorough understanding of these pathways is a critical step in the development of therapeutic treatments, antivirals, and vaccines.

Graphical Abstract
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Briant L, Despres P, Choumet V, Misse D. Role of skin immune cells on the host susceptibility to mosquito-borne viruses. Virology. 2014;464-465:26–32.
Surasombatpattana P, Hamel R, Patramool S, Luplertlop N, Thomas F, Desprès P, et al. Dengue virus replication in infected human keratinocytes leads to activation of antiviral innate immune responses. Infect Genet Evol. 2011;11(7):1664–73.
•• Pingen M, Bryden SR, Pondeville E, Schnettler E, Kohl A, Merits A, et al. Host inflammatory response to mosquito bites enhances the severity of arbovirus infection. Immunity. 2016;44(6):1455–69. This study shows that mosquito saliva injected into the skin during infection modulates the early immune response to arboviruses resulting in the recruitment of myeloid cells by neutrophils and promoting viral infection of recruited cells.
Hermance ME, Thangamani S. Proinflammatory cytokines and chemokines at the skin interface during Powassan virus transmission. J Investig Dermatol. 2014;134(8):2280–3.
Kurane I, Janus J, Ennis FA. Dengue virus infection of human skin fibroblasts in vitro production of IFN-beta, IL-6 and GM-CSF. Arch Virol. 1992;124(1–2):21–30.
Callaway JB, Smith SA, McKinnon KP, de Silva AM, Crowe JE Jr, Ting JP. Spleen tyrosine kinase (Syk) mediates IL-1beta induction by primary human monocytes during antibody-enhanced dengue virus infection. J Biol Chem. 2015;290(28):17306–20.
Brown MG, Hermann LL, Issekutz AC, Marshall JS, Rowter D, Al-Afif A, et al. Dengue virus infection of mast cells triggers endothelial cell activation. J Virol. 2011;85(2):1145–50.
Schneider BS, Higgs S. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans R Soc Trop Med Hyg. 2008;102(5):400–8.
Schneider BS, Soong L, Coffey LL, Stevenson HL, McGee CE, Higgs S. Aedes aegypti saliva alters leukocyte recruitment and cytokine signaling by antigen-presenting cells during West Nile virus infection. PLoS One. 2010;5(7):e11704.
McCracken MK, Christofferson RC, Chisenhall DM, Mores CN. Analysis of early dengue virus infection in mice as modulated by Aedes aegypti probing. J Virol. 2014;88(4):1881–9.
Styer LM, Lim PY, Louie KL, Albright RG, Kramer LD, Bernard KA. Mosquito saliva causes enhancement of West Nile virus infection in mice. J Virol. 2011;85(4):1517–27.
Teijaro JR. Type I interferons in viral control and immune regulation. Curr Opin Virol. 2016;16:31–40.
Pinto AK, Daffis S, Brien JD, Gainey MD, Yokoyama WM, Sheehan KC, et al. A temporal role of type I interferon signaling in CD8+ T cell maturation during acute West Nile virus infection. PLoS Pathog. 2011;7(12):e1002407.
Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, et al. A diverse array of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472(7344):481–5.
Miorin L, Maestre AM, Fernandez-Sesma A, Garcia-Sastre A. Antagonism of type I interferon by flaviviruses. Biochem Biophys Res Commun. 2017;492(4):587–96.
Aguirre S, Maestre AM, Pagni S, Patel JR, Savage T, Gutman D, et al. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS Pathog. 2012;8(10):e1002934.
Ashour J, Morrison J, Laurent-Rolle M, Belicha-Villanueva A, Plumlee CR, Bernal-Rubio D, et al. Mouse STAT2 restricts early dengue virus replication. Cell Host Microbe. 2010;8(5):410–21.
Grant A, Ponia SS, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, et al. Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe. 2016;19(6):882–90.
Yu C-Y, Chang T-H, Liang J-J, Chiang R-L, Lee Y-L, Liao C-L, et al. Dengue virus targets the adaptor protein MITA to subvert host innate immunity. PLoS Pathog. 2012;8(6):e1002780.
Calvert AE, Dixon KL, Delorey MJ, Blair CD, Roehrig JT. Development of a small animal peripheral challenge model of Japanese encephalitis virus using interferon deficient AG129 mice and the SA14-14-2 vaccine virus strain. Vaccine. 2014;32(2):258–64.
Chan KW, Watanabe S, Kavishna R, Alonso S, Vasudevan SG. Animal models for studying dengue pathogenesis and therapy. Antivir Res. 2015;123:5–14.
Julander JG. Animal models of yellow fever and their application in clinical research. Curr Opin Virol. 2016;18:64–9.
McGruder B, Saxena V, Wang T. Lessons from the murine models of West Nile virus infection. Methods Mol Biol(Clifton, NJ). 2016:1435, 61–9.
Morrison TE, Diamond MS. Animal models of Zika virus infection, pathogenesis, and immunity. J Virol. 2017;91(8).
Pinto AK, Brien JD, Lam C-YK, Johnson S, Chiang C, Hiscott J, et al. Defining new therapeutics using a more immunocompetent mouse model of antibody-enhanced dengue virus infection. mBio. 2015;6(5):e01316–5.
Malmgaard L. Induction and regulation of IFNs during viral infections. J Interf Cytokine Res. 2004;24(8):439–54.
Diamond MS, Harris E. Interferon inhibits dengue virus infection by preventing translation of viral RNA through a PKR-independent mechanism. Virology. 2001;289(2):297–311.
• Lam LKM, Watson AM, Ryman KD, Klimstra WB. Gamma-interferon exerts a critical early restriction on replication and dissemination of yellow fever virus vaccine strain 17D-204. NPJ Vaccines. 2018;3:5–5. This paper shows that IFNγ restricts both early dissemination and clearance of YF-17D. YF-17D is more susceptible to these effects than WT YFV suggesting that IFNγ sensitivity is one mechanism of attenuation of this vaccine.
Shrestha B, Wang T, Samuel MA, Whitby K, Craft J, Fikrig E, et al. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J Virol. 2006;80(11):5338–48.
Londono B, Colpitts T. A Brief Review of West Nile Virus Biology. 2016.
Best SM, Morris KL, Shannon JG, Robertson SJ, Mitzel DN, Park GS, et al. Inhibition of interferon-stimulated JAK-STAT signaling by a tick-borne flavivirus and identification of NS5 as an interferon antagonist. J Virol. 2005;79(20):12828–39.
Muñoz-Jordán JL, Sánchez-Burgos GG, Laurent-Rolle M, García-Sastre A. Inhibition of interferon signaling by dengue virus. Proc Natl Acad Sci. 2003;100(24):14333–8.
• Chaudhary V, Yuen KS, Chan JF, Chan CP, Wang PH, Cai JP, et al. Selective activation of type ii interferon signaling by Zika virus ns5 protein. J Virol. 2017;91(14). This paper shows that ZIKV NS5 protein enhances IFNγ signaling by promoting Stat1 homodimerization and recruitment to ISG promoters. Interestingly, this has a positive effect on ZIKV replication.
Palomo J, Dietrich D, Martin P, Palmer G, Gabay C. The interleukin (IL)-1 cytokine family--balance between agonists and antagonists in inflammatory diseases. Cytokine. 2015;76(1):25–37.
• Aarreberg LD, Wilkins C, Ramos HJ, Green R, Davis MA, Chow K, et al. Interleukin-1beta signaling in dendritic cells induces antiviral interferon responses. mBio. 2018;9(2). This study uses global transcriptome analysis to address the role of IL-1R signaling in primary DCs and macrophages during WNV infection. Their data suggest that for effective clearance of WNV, cross-talk between IL-1β and IFNβ is required.
Wu MF, Chen ST, Yang AH, Lin WW, Lin YL, Chen NJ, et al. CLEC5A is critical for dengue virus-induced inflammasome activation in human macrophages. Blood. 2013;121(1):95–106.
Chang D-M, Shaio M-F. Production of interleukin-1 (IL-1) and IL-1 inhibitor by human monocytes exposed to dengue virus. J Infect Dis. 1994;170(4):811–7.
Barros VE, Ferreira BR, Livonesi M, Figueiredo LT. Cytokine and nitric oxide production by mouse macrophages infected with Brazilian flaviviruses. Rev Inst Med Trop Sao Paulo. 2009;51(3):141–7.
Avendano-Tamayo E, Campo O, Chacon-Duque JC, Ramirez R, Rojas W, Agudelo-Florez P, et al. Variants in the TNFA, IL6 and IFNG genes are associated with the dengue severity in a sample from Colombian population. Biomedica: revista del Instituto Nacional de Salud- Biomedica. 2017;37(4):486–97.
Eppy S, Nainggolan L, Rumende CM. The differences between Interleukin-6 and C-reactive protein levels among adult patients of dengue infection with and without plasma leakage. Acta Med Indones. 2016;48(1):3–9.
Iani FC, Caldas S, Duarte MM, Cury AL, Cecilio AB, Costa PA, et al. Dengue patients with early hemorrhagic manifestations lose coordinate expression of the anti-inflammatory cytokine IL-10 with the inflammatory cytokines IL-6 and IL-8. Am J Trop Med Hyg. 2016;95(1):193–200.
Meier KC, Gardner CL, Khoretonenko MV, Klimstra WB, Ryman KD. A mouse model for studying viscerotropic disease caused by yellow fever virus infection. PLoS Pathog. 2009;5(10):e1000614.
ter Meulen J, Sakho M, Koulemou K, Magassouba N, Bah A, Preiser W, et al. Activation of the cytokine network and unfavorable outcome in patients with yellow fever. J Infect Dis. 2004;190(10):1821–7.
•• Inyoo S, Suttitheptumrong A, Pattanakitsakul SN. Synergistic effect of TNF-alpha and dengue virus infection on adhesion molecule reorganization in human endothelial cells. Jpn J Infect Dis. 2017;70(2):186–91. This paper shows that treatment of human endothelial cells with TNFα and DENV leads to reorganization of adherence and junction proteins which correlates with increased permeability, adding to our understanding of the mechanisms involved in vascular leakage during DENV severe disease.
Fernandez-Mestre MT, Gendzekhadze K, Rivas-Vetencourt P, Layrisse Z. TNF-alpha-308A allele, a possible severity risk factor of hemorrhagic manifestation in dengue fever patients. Tissue Antigens. 2004;64(4):469–72.
Pujhari SK, Ratho RK, Prabhakar S, Mishra B, Modi M. TNF-α promoter polymorphism: a factor contributing to the different immunological and clinical phenotypes in Japanese encephalitis. BMC Infect Dis [Internet]. 2012 2012; 12.
Sanchez-Leyva M, Sanchez-Zazueta JG, Osuna-Ramos JF, Rendon-Aguilar H, Felix-Espinoza R, Becerra-Loaiza DS, et al. Genetic polymorphisms of tumor necrosis factor alpha and susceptibility to dengue virus infection in a Mexican population. Viral Immunol. 2017;30(8):615–21.
Pinto AK, Ramos HJ, Wu X, Aggarwal S, Shrestha B, Gorman M, et al. Deficient IFN signaling by myeloid cells leads to MAVS-dependent virus-induced sepsis. PLoS Pathog. 2014;10(4):e1004086.
Shrestha B, Zhang B, Purtha WE, Klein RS, Diamond MS. Tumor necrosis factor alpha protects against lethal West Nile virus infection by promoting trafficking of mononuclear leukocytes into the central nervous system. J Virol. 2008;82(18):8956–64.
Tun MMN, Aoki K, Senba M, Buerano CC, Shirai K, Suzuki R, et al. Protective role of TNF-α, IL-10 and IL-2 in mice infected with the Oshima strain of Tick-borne encephalitis virus. Sci Rep [Internet]. 2014;4:5344.
Lannes N, Summerfield A, Filgueira L. Regulation of inflammation in Japanese encephalitis. J Neuroinflammation. 2017;14(1):158.
Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–81.
Woodson SE, Freiberg AN, Holbrook MR. Differential cytokine responses from primary human Kupffer cells following infection with wild-type or vaccine strain yellow fever virus. Virology. 2011;412(1):188–95.
Foo SS, Chen W, Chan Y, Bowman JW, Chang LC, Choi Y, et al. Asian Zika virus strains target CD14(+) blood monocytes and induce M2-skewed immunosuppression during pregnancy. Nat Microbiol. 2017;2(11):1558–70.
Tauseef A, Umar N, Sabir S, Akmal A, Sajjad S, Zulfiqar S. Interleukin-10 as a marker of disease progression in dengue hemorrhagic fever. J Coll Physicians Surg Pak. 2016;26(3):187–90.
Bai F, Town T, Qian F, Wang P, Kamanaka M, Connolly TM, et al. IL-10 signaling blockade controls murine West Nile virus infection. PLoS Pathog. 2009;5(10):e1000610.
Suthar MS, Diamond MS, Gale M Jr. West Nile virus infection and immunity. Nat Rev Microbiol. 2013;11(2):115–28.
Duangchinda T, Dejnirattisai W, Vasanawathana S, Limpitikul W, Tangthawornchaikul N, Malasit P, et al. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc Natl Acad Sci U S A. 2010;107(39):16922–7.
Dung NT, Duyen HT, Thuy NT, Ngoc TV, Chau NV, Hien TT, et al. Timing of CD8+ T cell responses in relation to commencement of capillary leakage in children with dengue. J Immunol (Baltimore, Md : 1950). 2010;184(12):7281–7.
Weiskopf D, Angelo MA, de Azeredo EL, Sidney J, Greenbaum JA, Fernando AN, et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci U S A. 2013;110(22):E2046–53.
Hassert M, Brien JD, Pinto AK. CD4+T cells mediate protection against Zika associated severe disease in a mouse model of infection. PLoS Pathog. 2018. https://doi.org/10.1371/journal.ppat.1007237.
Fagundes CT, Costa VV, Cisalpino D, Amaral FA, Souza PRS, Souza RS, et al. IFN-γ production depends on IL-12 and IL-18 combined action and mediates host resistance to dengue virus infection in a nitric oxide-dependent manner. PLoS Negl Trop Dis. 2011;5(12):e1449.
Robertson SJ, Lubick KJ, Freedman BA, Carmody AB, Best SM. Tick-borne flaviviruses antagonize both IRF-1 and type I interferon signaling to inhibit dendritic cell function. J Immunol (Baltimore, Md : 1950). 2014;192(6):2744–55.
Brien JD, Uhrlaub JL, Nikolich-Zugich J. West Nile virus-specific CD4 T cells exhibit direct antiviral cytokine secretion and cytotoxicity and are sufficient for antiviral protection. J Immunol (Baltimore, Md : 1950). 2008;181(12):8568–75.
Tappe D, Perez-Giron JV, Zammarchi L, Rissland J, Ferreira DF, Jaenisch T, et al. Cytokine kinetics of Zika virus-infected patients from acute to reconvalescent phase. Med Microbiol Immunol. 2016;205(3):269–73.
Cimini E, Castilletti C, Sacchi A, Casetti R, Bordoni V, Romanelli A, et al. Human Zika infection induces a reduction of IFN-γ producing CD4 T-cells and a parallel expansion of effector Vδ2 T-cells. Sci Rep [Internet]. 2017;7(1):6313.
Senaratne T, Carr J, Noordeen F. Elevation in liver enzymes is associated with increased IL-2 and predicts severe outcomes in clinically apparent dengue virus infection. Cytokine. 2016;83:182–8.
Das S, Khader S. Yin and yang of interleukin-17 in host immunity to infection. F1000Research. 2017;6:741.
Jain A, Pandey N, Garg RK, Kumar R. IL-17 level in patients with dengue virus infection & its association with severity of illness. J Clin Immunol. 2013;33(3):613–8.
Guabiraba R, Besnard AG, Marques RE, Maillet I, Fagundes CT, Conceicao TM, et al. IL-22 modulates IL-17A production and controls inflammation and tissue damage in experimental dengue infection. Eur J Immunol. 2013;43(6):1529–44.
Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol (Baltimore, Md : 1950). 2005;175(6):3463–8.
Lin YW, Wang KJ, Lei HY, Lin YS, Yeh TM, Liu HS, et al. Virus replication and cytokine production in dengue virus-infected human B lymphocytes. J Virol. 2002;76(23):12242–9.
• Liu H, Wu R, Liu K, Yuan L, Huang X, Wen Y, et al. Enhanced immune responses against Japanese encephalitis virus using recombinant adenoviruses coexpressing Japanese encephalitis virus envelope and porcine interleukin-6 proteins in mice. Virus Res. 2016;222:34–40. This study shows the efficacy of recombinant adenovirus expressing JEV envelope epitopes with IL-6 in mice as a vaccine against JEV. The results present evidence that IL-6 is a strong adjuvant that can enhance humoral and cellular immune responses in the context of JEV vaccination.
Vivanco-Cid H, Maldonado-Renteria MJ, Sanchez-Vargas LA, Izaguirre-Hernandez IY, Hernandez-Flores KG, Remes-Ruiz R. Dynamics of interleukin-21 production during the clinical course of primary and secondary dengue virus infections. Immunol Lett. 2014;161(1):89–95.
Kuchen S, Robbins R, Sims GP, Sheng C, Phillips TM, Lipsky PE, et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4 T cell-B cell collaboration. J Immunol. 2007;179(9):5886–96.
Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177(8):5236–47.
Jin H, Carrio R, Yu A, Malek TR. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J Immunol. 2004;173(1):657–65.
Ozaki K, Spolski R, Feng CG, Qi C-F, Cheng J, Sher A, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298(5598):1630–4.
• Douam F, Soto Albrecht YE, Hrebikova G, Sadimin E, Davidson C, Kotenko SV, et al. Type III interferon-mediated signaling is critical for controlling live attenuated yellow fever virus infection in vivo. mBio. 2017;8(4). In this paper, the authors show that type III IFN is critical for controlling YF-17D and preserving attenuation in a mouse model of infection through maintaining the BBB integrity and preventing neuroinvasion.
Lazear HM, Daniels BP, Pinto AK, Huang AC, Vick SC, Doyle SE, et al. Interferon-lambda restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci Transl Med. 2015;7(284):284ra59.
Calvet G, Aguiar RS, Melo ASO, Sampaio SA, de Filippis I, Fabri A, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016;16(6):653–60.
Martines RB, Bhatnagar J, de Oliveira Ramos AM, Davi HPF, Iglezias SDA, Kanamura CT, et al. Pathology of congenital Zika syndrome in Brazil: a case series. Lancet. 2016;388(10047):898–904.
• Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET Jr, et al. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe. 2016;19(5):705–12. This study shows that primary human placental trophoblasts secrete IFNλ which functions in an autocrine and paracrine fashion to protect susceptible cells from ZIKV infection.
•• Chen J, Liang Y, Yi P, Xu L, Hawkins HK, Rossi SL, et al. Outcomes of congenital Zika disease depend on timing of infection and maternal-fetal interferon action. Cell Rep. 2017;21(6):1588–99. This paper shows protective effects of IFNλ treatment of pregnant mice during ZIKV infection and suggests IFNλ treatment as a potential therapeutic for pregnant women at risk for congenital Zika disease.
• Corry J, Arora N, Good CA, Sadovsky Y, Coyne CB. Organotypic models of type III interferon-mediated protection from Zika virus infections at the maternal–fetal interface. Proc Natl Acad Sci. 2017;114(35):9433–8. The authors of this paper develop 3D culture models of human syncytiotrophoblasts, which produce type III IFNs to confer resistance to ZIKV infection. Syncytiotrophoblasts isolated from the second trimester constitutively express type III IFNs which provides some mechanistic insight into the maternal mechanisms of protection of the fetus.
• Jagger BW, Miner JJ, Cao B, Arora N, Smith AM, Kovacs A, et al. Gestational stage and IFN-lambda signaling regulate ZIKV infection in utero. Cell Host Microbe. 2017;22(3):366–76.e3. In this paper, the authors highlight the kinetics in production of type III IFN and its function in the protection of the fetus in a mouse model of congenital ZIKV infection, supporting the findings of Bayer et. al., Chen et. al., and Corry et. al..
Ramos HJ, Lanteri MC, Blahnik G, Negash A, Suthar MS, Brassil MM, et al. IL-1beta signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog. 2012;8(11):e1003039.
Kumar M, Roe K, Orillo B, Muruve DA, Nerurkar VR, Gale M Jr, et al. Inflammasome adaptor protein apoptosis-associated speck-like protein containing CARD (ASC) is critical for the immune response and survival in West Nile virus encephalitis. J Virol. 2013;87(7):3655–67.
Chen C-J, Ou Y-C, Li J-R, Chang C-Y, Pan H-C, Lai C-Y, et al. Infection of pericytes in vitro by Japanese encephalitis virus disrupts the integrity of the endothelial barrier. J Virol. 2014;88(2):1150–61.
Town T, Bai F, Wang T, Kaplan AT, Qian F, Montgomery RR, et al. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity. 2009;30(2):242–53.
Hayasaka D, Shirai K, Aoki K, Nagata N, Simantini DS, Kitaura K, et al. TNF-α acts as an immunoregulator in the mouse brain by reducing the incidence of severe disease following Japanese encephalitis virus infection. PLoS One. 2013;8(8):e71643.
Yun SI, Lee YM. Japanese encephalitis: the virus and vaccines. Human vaccines & immunotherapeutics. 2014;10(2):263–79.
Japanese Encephalitis Vaccines. WHO position paper, February 2015--recommendations. Vaccine. 2016;34(3):302–3.
Samaan Z, McDermid Vaz S, Bawor M, Potter TH, Eskandarian S, Loeb M. Neuropsychological impact of West Nile virus infection: an extensive neuropsychiatric assessment of 49 cases in Canada. PLoS One. 2016;11(6):e0158364.
Czupryna P, Moniuszko A, Pancewicz SA, Grygorczuk S, Kondrusik M, Zajkowska J. Tick-borne encephalitis in Poland in years 1993-2008--epidemiology and clinical presentation. A retrospective study of 687 patients. Eur J Neurol. 2011;18(5):673–9.
•• Garber C, Vasek MJ, Vollmer LL, Sun T, Jiang X, Klein RS. Astrocytes decrease adult neurogenesis during virus-induced memory dysfunction via IL-1. Nat Immunol. 2018;19(2):151–61. The authors of this study use transcriptional profiling to implicate IL-1β in poor recovery in a mouse model of WNV-induced cognitive dysfunction. The authors show that IL-1β produced by astrocytes led to impaired neuronal progenitor cell development which lends insight into the mechanism underlying long-term sequela associated with WNV infection.
Vasek MJ, Garber C, Dorsey D, Durrant DM, Bollman B, Soung A, et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534(7608):538–43.
Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–57.
Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (London, England : 1979). 2012;122(4):143–59.
Garcia MN, Hause AM, Walker CM, Orange JS, Hasbun R, Murray KO. Evaluation of prolonged fatigue post–West Nile virus infection and association of fatigue with elevated antiviral and proinflammatory cytokines. Viral Immunol. 2014;27(7):327–33.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Brien and Dr. Pinto reports grants from Emergent Biosolutions, outside the submitted work; .
Dr. Hassert has no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Virology
Rights and permissions
About this article
Cite this article
Hassert, M., Brien, J.D. & Pinto, A.K. The Temporal Role of Cytokines in Flavivirus Protection and Pathogenesis. Curr Clin Micro Rpt 6, 25–33 (2019). https://doi.org/10.1007/s40588-018-0106-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-018-0106-x