The atrial secondary tricuspid regurgitation is associated to more favorable outcome than the ventricular phenotype
- 1Department of Cardiology, Istituto Auxologico Italiano, IRCCS, Milan, Italy
- 2Department of Medicine and Surgery, University of Milano-Bicocca, Milan, Italy
- 3Emergency University Hospital Bucharest, University of Medicine and Pharmacy Carol Davila Bucharest, Bucharest, Romania
- 4Department of Management, Information, and Production Engineering, University of Bergamo, Dalmine, Italy
Aim: We sought to evaluate the differences in prognosis between the atrial (A-STR) and the ventricular (V-STR) phenotypes of secondary tricuspid regurgitation.
Materials and methods: Consecutive patients with moderate or severe STR referred for echocardiography were enrolled. A-STR and V-STR were defined according to the last ACC/AHA guidelines criteria. The primary endpoint was the composite of all-cause death and heart failure (HF) hospitalizations.
Results: A total of 211 patients were enrolled. The prevalence of A-STR in our cohort was 26%. Patients with A- STR were significantly older and with lower NYHA functional class than V-STR patients. The prevalence of severe STR was similar (28% in A-STR vs. 37% in V-STR, p = 0.291). A-STR patients had smaller tenting height (TH) (10 ± 4 mm vs. 12 ± 7 mm, p = 0.023), larger end-diastolic tricuspid annulus area (9 ± 2 cm2 vs. 7 ± 6 cm2/m2, p = 0.007), smaller right ventricular (RV) end-diastolic volumes (72 ± 27 ml/m2 vs. 92 ± 38 ml/m2; p = 0.001), and better RV longitudinal function (18 ± 7 mm vs. 16 ± 6 mm; p = 0.126 for TAPSE, and −21 ± 5% vs. −18 ± 5%; p = 0.006, for RV free-wall longitudinal strain, RVFWLS) than V-STR patients. Conversely, RV ejection fraction (RVEF, 48 ± 10% vs. 46 ± 11%, p = 0.257) and maximal right atrial volumes (64 ± 38 ml/m2 vs. 55 ± 23 ml/m2, p = 0.327) were similar between the two groups. After a median follow-up of 10 months, patients with V-STR had a 2.7-fold higher risk (HR: 2.7, 95% CI 95% = 1.3–5.7) of experiencing the combined endpoint than A-STR patients. The factors related to outcomes resulted different between the two STR phenotypes: TR-severity (HR: 5.8, CI 95% = 1, 4–25, P = 0.019) in A-STR patients; TR severity (HR 2.9, 95% CI 1.4–6.3, p = 0.005), RVEF (HR: 0.97, 95% CI 0.94–0.99, p = 0.044), and RVFWLS (HR: 0.93, 95% CI 0.85–0.98, p = 0.009) in V-STR.
Conclusion: Almost one-third of patients referred to the echocardiography laboratory for significant STR have A-STR. A-STR patients had a lower incidence of the combined endpoint than V-STR patients. Moreover, while TR severity was the only independent factor associated to outcome in A-STR patients, TR severity and RV function were independently associated with outcome in V-STR patients.
Introduction
Secondary tricuspid regurgitation (STR) represents more than 90% of clinically relevant TR (1, 2) and is defined as TR that occurs in structurally normal tricuspid valve (TV) leaflets whose etiology is ascribed to tricuspid annular (TA) dilatation with or without leaflet tethering (3, 4).
Although the most common and well-known etiology of STR is the right ventricular (RV) dysfunction secondary to left-sided heart disease and pulmonary hypertension (PH), “isolated-TR” (TR not related to left-side heart disease or to significant pulmonary artery hypertension) has recently emerged as an important “phenotype” of STR. The prevalence of this phenotype of STR is growing because of the aging of the general population (1), and it develops mostly because of the right atrial (RA) dilatation associated with atrial fibrillation (AF) (5–8). The clarification of the pathogenic connection with AF and tricuspid annulus (TA) dilatation (8–10) has meant that this form of STR is currently defined as “atrial” STR (A-STR) (11).
Not surprisingly, the most recent guidelines about the management of heart valve diseases suggest that A-STR should be separated from the other forms of STR (12, 13) since the mechanisms underlying these two STR phenotypes have been hypothesized to be different (14). Notwithstanding, the recently published studies, including STR patients, do not distinguish between the A-STR and the “ventricular” STR (V-STR) phenotypes (15–19) and, to the best of our knowledge, only one echocardiographic study examined the differences in geometry of the right heart structures between patients with A-STR and V-STR (20). Furthermore, since A-STR has long been under-recognized as a distinct phenotype of STR, its prognosis and prognostic predictors remain to be clarified, especially in comparison with V-STR, in which the prognosis is also associated with the underlying RV function and/or associated left heart conditions (18, 19, 21). Accordingly, we tested the hypothesis that the prognosis and the prognostic correlates differ between patients with A-STR and V-STR.
Materials and methods
Study population and outcomes
Consecutive patients referred for echocardiography between 2016 and 2021 with the first diagnosis of moderate or severe STR were included in a prospective observational study (FUTURE 3DECHO). According to the most recent guidelines of the American Heart Association/American College of Cardiology (12), patients with moderate to severe STR were classified as A-STR when having AF, left ventricular ejection fraction > 60%, pulmonary artery systolic pressure (PASP) < 50 mm Hg, no left-sided valve disease, and normal-appearing tricuspid valve leaflets. Patients with STR not fitting all these three criteria were defined as having ventricular STR (V-STR) (12). Informed consent was collected from all subjects and the study was approved by the Istituto Auxologico Ethics Committee (record #2020_04_21_06, approved on April 21, 2020). The data collection and the echocardiographic analysis, as well as the follow-up, were performed by trained echocardiographers according to the best clinical practice and following the most recent recommendations (4, 22–24). Exclusion criteria were: poor echocardiographic image quality, a pacemaker or implantable cardioverter-defibrillator, organic tricuspid regurgitation, highly irregular cardiac rhythm (precluding the acquisition of multi-beat 3DE datasets with no stitching artifacts), pulmonary valve relevant pathologies or pulmonary artery stenosis; planned TV surgery or transcatheter intervention in the 3 months following the echocardiography evaluation.
Echocardiography
Study subjects underwent standard 2DE and Doppler studies using commercially available Vivid E9/E95 systems (GE Vingmed, Horten, Norway) equipped with M5S probe. In addition, multi-beat 3D datasets of the RA, TV, and RV were acquired from the apical approach using the 4V/4Vc probes. Images were digitally stored and analyzed offline using either EchoPAC 202 or 204 (GE Vingmed, Horten, Norway) by a single experienced researcher blinded to the medical history of the patients. Left ventricular (LV) volumes, LV ejection fraction (LVEF), PASP, and LV diastolic function were assessed in all the patients according to the most recent recommendations (4, 22–25). Conventional 2DE parameters of RA and RV size and function were obtained from the RV-focused apical view. 2DE TA diameter was measured from both the apical 4-chamber view and the RV-focused apical view at end-diastole (identified as the frame before the TV closure). RV free-wall longitudinal strain (RVFWLS) and 4-chamber RV strain (RV4CHLS) were obtained from the RV-focused apical view according to the current recommendations (26, 27). TR severity was graded as mild, moderate, or severe using the recommended multiparametric approach, which included: the average vena contracta (VC) width (measured in apical RV-focused and parasternal long-axis RV inflow views), the proximal isovelocity surface area (PISA) radius of the regurgitant jet at a Nyquist limit of 29 cm/s, the effective regurgitant orifice area (EROA) (23–25, 28, 29). To calculate the EROA and the regurgitant volume we used the PISA formula corrected for the TV leaflet tethering angle and the TR flow velocity (29). Full-volume 3DE acquisitions of the RV, TV, and RA were obtained from the RV-focused apical view using electrocardiogram gating over 4 to 6 consecutive cardiac cycles during a single breath-hold. Gain settings were optimized, and the sector width and the image depth were adjusted to maximize the temporal resolution. The RV end-diastolic (EDV) and end-systolic (ESV) volumes, and the RV ejection fraction (RVEF) were measured using 4D Auto RVQ (EchoPac 202 and 204, GE Vingmed, Horten, NO) (30). The absence of structural tricuspid valve (TV) diseases was checked by obtaining multiple cut-planes from the volume-rendered 3DE dataset of the valve. Finally, the size and the shape of the TA and leaflets’ coaptation position were evaluated using a dedicated software package (4D Auto TVQ, EchoPac v204, GE, Horten, Norway) (5, 26, 27; Figure 1).
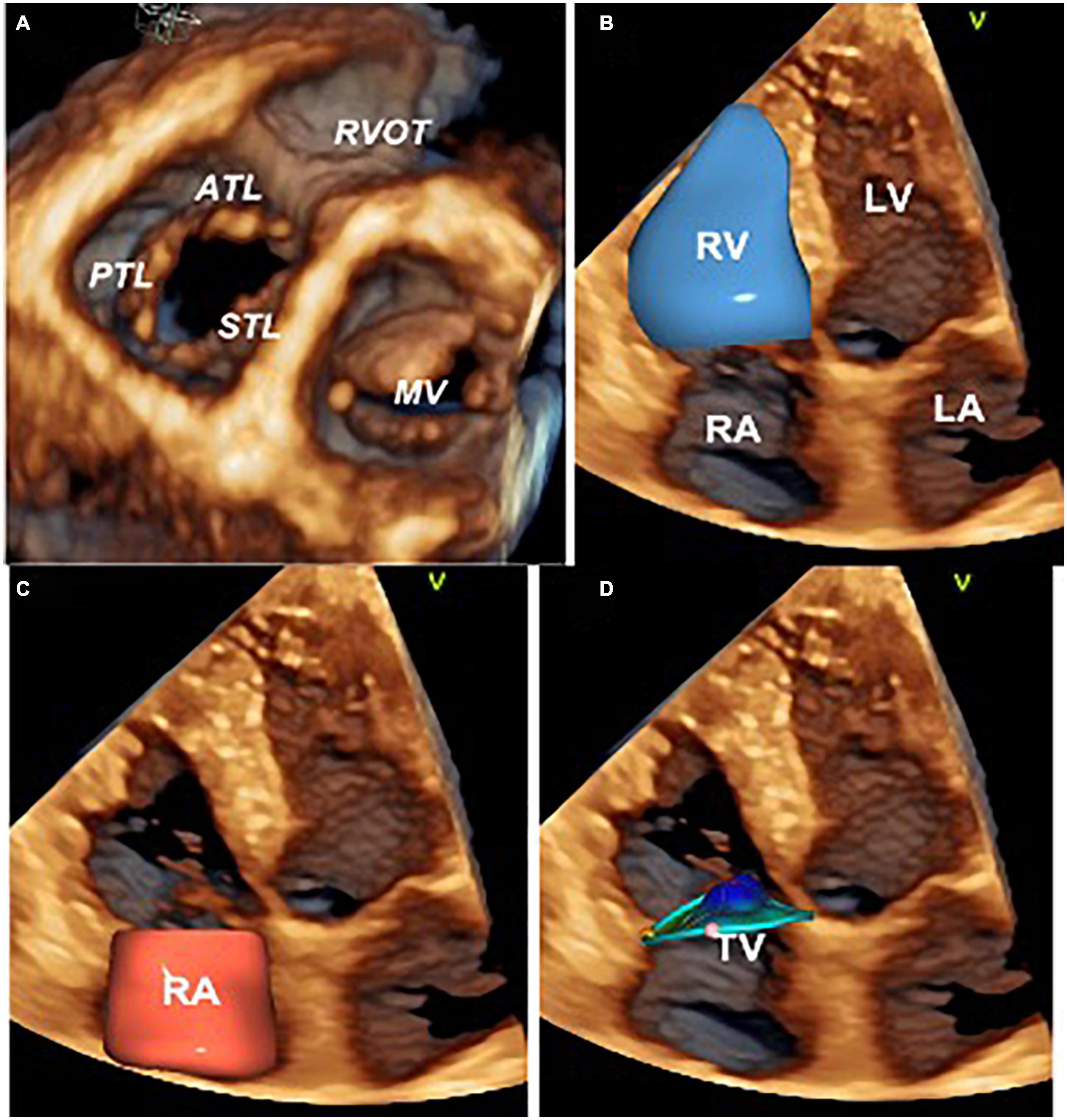
Figure 1. Three-dimensional echocardiography assessment of the tricuspid valve, right heart chambers, and tricuspid annulus. Ventricular view of the tricuspid and mitral valve leaflets by transthoracic three-dimensional echocardiography (A). Surface rendering and volumetric analysis of the right ventricle (B) and right atrium (C). Surface rendering and measurement of the tricuspid annulus size and shape with 4D AutoTVQ software package (GE Vingmed, Horten, NO) (D). ATL, anterior tricuspid leaflet; LA, left atrium; LV, left ventricle; MV, mitral valve; RVOT, right ventricle outflow tract; PTL, posterior tricuspid leaflet; RV, right ventricle; RA, right atrium; STL, septal tricuspid leaflet; TV, tricuspid valve.
Follow-up and study endpoint
The primary endpoint of the study was the occurrence of death for any cause and/or hospitalization for heart failure. Information concerning survival and hospitalization were obtained at regular intervals through (i) telephone interviews with the patient, or if deceased, with family members; (ii) contact with the patient’s physician(s); and (iii) review of electronic medical records of regular outpatient visits and hospital admission records. Mortality status was verified independently through the Social Security Death Index and death certificates. Assignment of clinical events was performed by physicians unaware of the patients’ echocardiographic and clinical characteristics. For patients without events, the date of the last contact was used for survival analysis.
Statistical analysis
The normal distribution of continuous variables was tested with Kolmogorov–Smirnov test. Continuous variables were reported as mean ± standard deviation (SD) and were compared using either the Student’s t-test or the Mann–Whitney test. Categorical variables were reported as counts and percentages and compared using the Fisher’s exact tests, as appropriate. Cox regression models were used to estimate the unadjusted and adjusted relative risk of clinical endpoints at follow-up. The results were shown as the hazard ratio (HR) with the corresponding 95% CI. Before running the multivariable analysis, we tested the correlation between echocardiographic parameters related to prognosis with the Pearson coefficient to exclude any multicollinearity. We included in the multivariable analysis the echocardiographic factors that resulted to be related to the survival at univariate analysis selected on the basis of their interaction and clinical relevance (i.e., p < 0.05 at univariate analysis). The cumulative incidence of all-cause mortality or HF hospitalization was estimated using the Kaplan–Meier method. All statistical analyses were performed using the SPSS software, version 20 (SPSS Inc., Chicago, IL, USA). A two-sided significance level of p < 0.05 was considered statistically significant.
Results
Clinical characteristics, two- and three-dimensional echocardiography parameters
A total of 211 patients were included (Figure 2). Table 1 summarizes the clinal characteristics of our patients and the differences between patients with A-STR and V-STR. Compared with V-STR patients, A-STR patients (n = 26% of the study population) were significantly older and with a lower NYHA functional class. The prevalence of both ischemic heart disease and chronic obstructive pulmonary disease was higher in V-STR patients (Table 1).
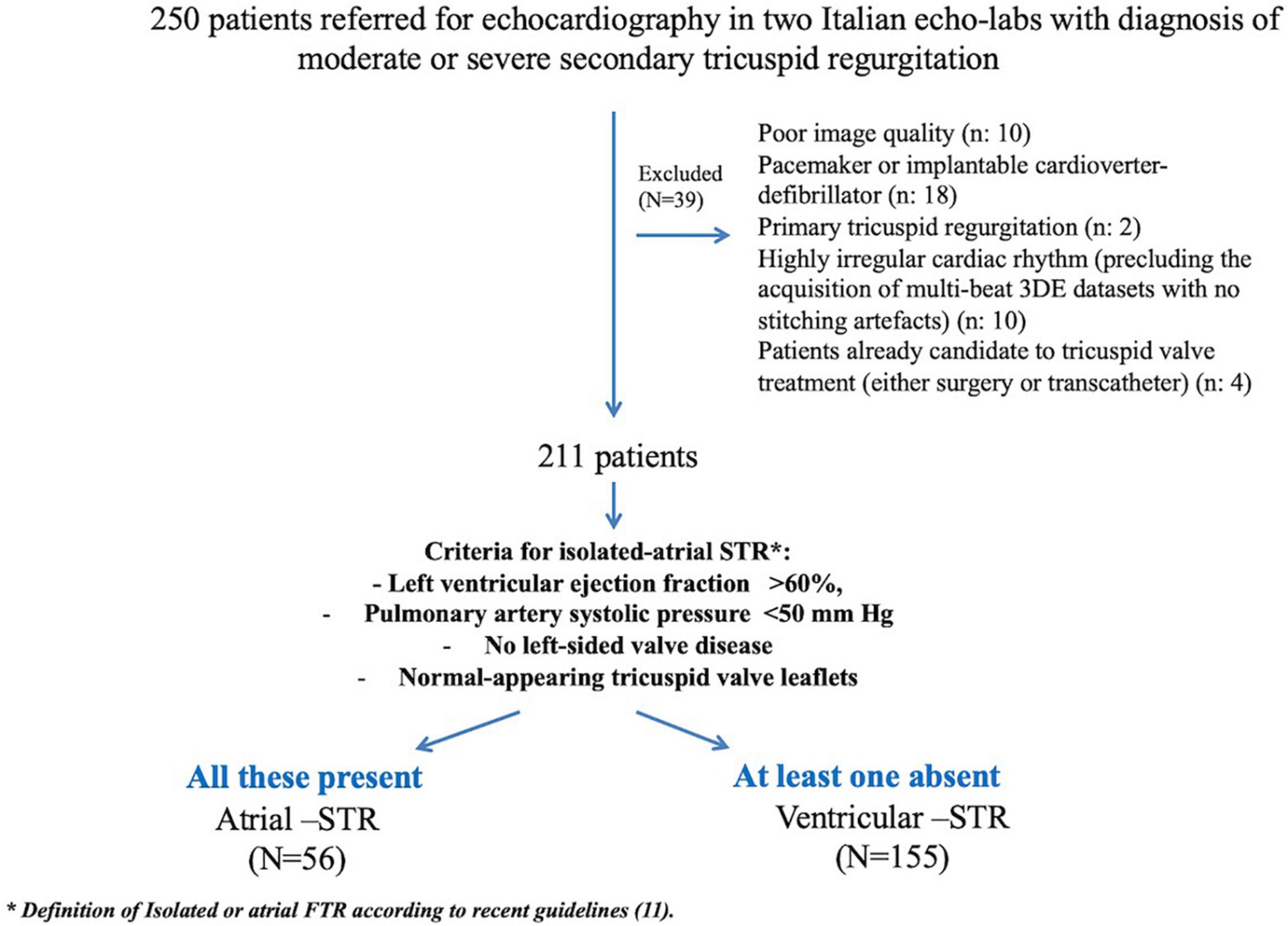
Figure 2. Study flow-chart. 3DE, three-dimensional echocardiography; A-STR, atrial secondary tricuspid regurgitation; RV, right ventricular; V-STR, ventricular secondary tricuspid regurgitation.
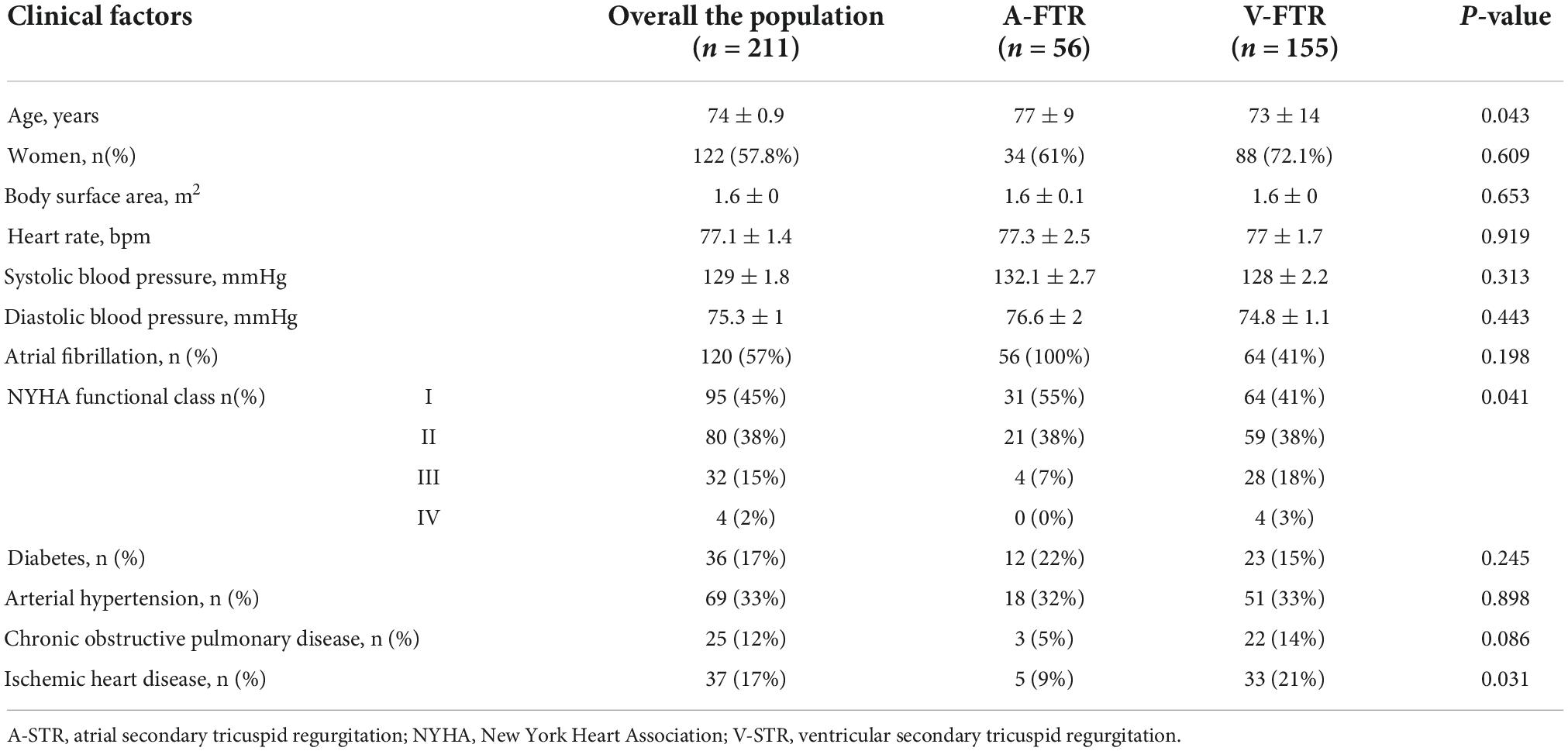
Table 1. Clinical characteristics of patients with atrial and ventricular secondary tricuspid regurgitation.
Table 2 summarizes the echocardiographic characteristics of our patients and the differences between patients with A-STR and V-STR. LVEF was higher in A-STR than in V-STR patients, and 7% of V-STR patients had LVEF < 40%. As expected, PASP was lower in A-STR patients (Table 2). The prevalence of severe STR was similar between A-STR and V-STR patients. However, V-STR patients showed larger vena contracta size (both the vena contracta width measured by 2DE and the vena contracta area measured by 3DE, Table 2). Moreover, TV remodeling parameters were significantly different between the two phenotypes. Patients with A-STR showed smaller tenting height and larger 3D end-diastolic tricuspid annulus area than V-STR patients (Table 2). RA volumes were similarly enlarged in A-STR and V-STR patients. The same was true for the RV basal end-diastolic diameter. Conversely, RV volumes were smaller in A-STR than in V-STR (Table 2). Global RV function (i.e., RVEF and TAPSE) were similar in A-STR and V-STR patients. Conversely, RV myocardial longitudinal deformation (reported as RVFWLS and RV4CLS) resulted significantly better in A-STR than in V-STR (Table 2). Similarly, RV-pulmonary artery coupling estimated by both TAPSE/PASP and RVFWLS/PASP was better in A-STR than in V-STR (Table 2).
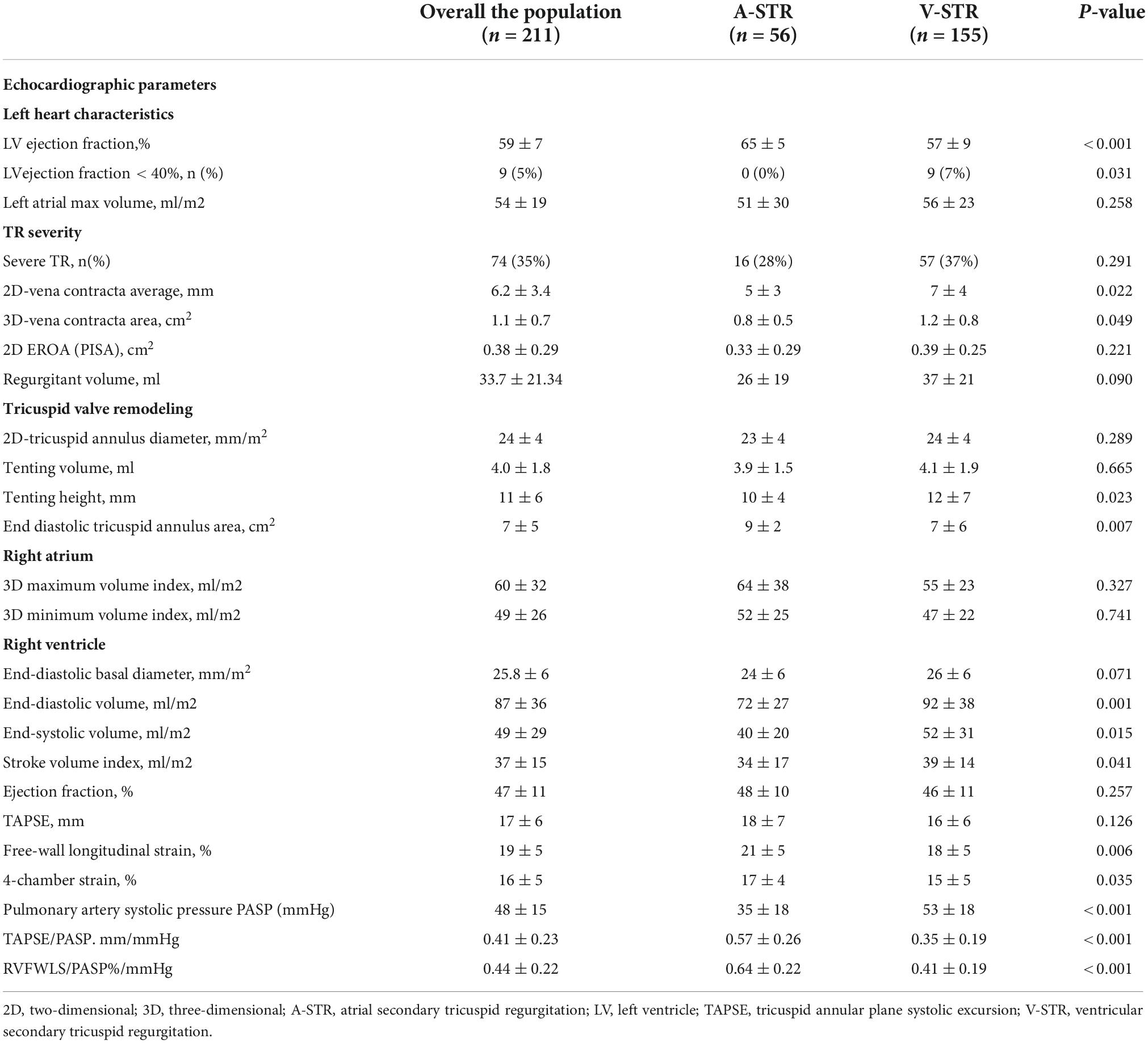
Table 2. Echocardiographic characteristics of patients with atrial and ventricular secondary tricuspid regurgitation.
Clinical outcomes and associated echocardiography factors
After a median follow-up of 10 months (IQR: 2–23), the rate of the composite endpoint was significantly different in the two groups (14% A-STR vs. 39% V-STR, p = 0.004), with a 2.15-fold significantly higher risk of 1 year combined endpoint for V-STR patients than A-STR (Figure 3).
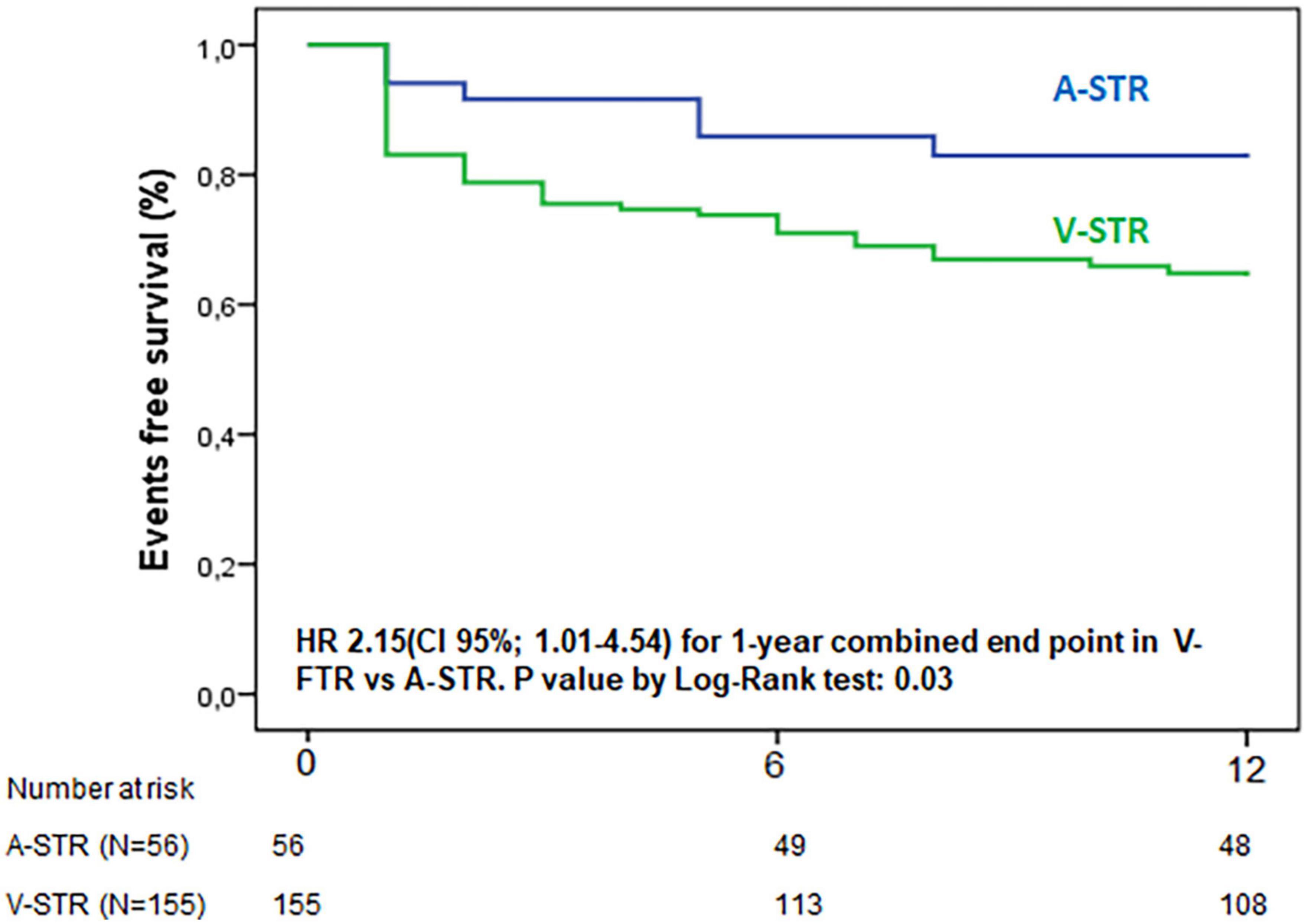
Figure 3. Kaplan-Meier curves for 1 year combined endpoint of all-cause death and hospitalization for heart failure. A-STR, atrial-secondary tricuspid regurgitation; HR, hazard Ratio; V-STR, ventricular secondary tricuspid regurgitation.
Univariate Cox regression was performed for the whole study population, and then, separately, for each phenotype of STR to test the correlates of clinical outcomes (Table 3). All the indexes of TR severity were related to the composite endpoint in both groups. TH was associated with outcomes only in V-STR patients (P = 0.003). Conversely, the end-diastolic TA area was associated with outcomes only in A-STR (P = 0.025). Both RV end-diastolic and RA volumes were associated with outcomes at univariate analysis both in A-STR and V-STR patients. However, the RV function parameters were associated with outcomes only in V-STR patients (Table 3). Before running the multivariable regression analysis, we tested the correlation between echocardiographic variables. In A-STR patients, 3D maximal RA volume was related to the 3D end-diastolic TA area and the RV end-diastolic volume was related to the EROA. Accordingly, we included only TR severity and 3D maximal RA volume in our multivariable regression model, and TR severity resulted independently associated with the combined endpoint (HR: 5.8, CI 95%: 1.4–25, P = 0.019). In V-STR patients, a close correlation was found between 3D maximal RA volume and EROA, RV end-diastolic volume and TAPSE, and between RVEF, TAPSE, and TAPSE/PASP. Therefore, only TR severity, RVFWLS, RVEF, and 3D maximal RA volume were included in the final multivariable regression model (Table 4). TR severity (HR 2.9, CI 95% 1.4–6.3, P = 0.005 for TR severe), RVEF (HR: 0.97, CI 95%: 0.94–0.99, P = 0.044), and RVFWLS (HR: 0.93, CI 95%: 0.85–0.98, P = 0.009) resulted independently associated with outcomes.
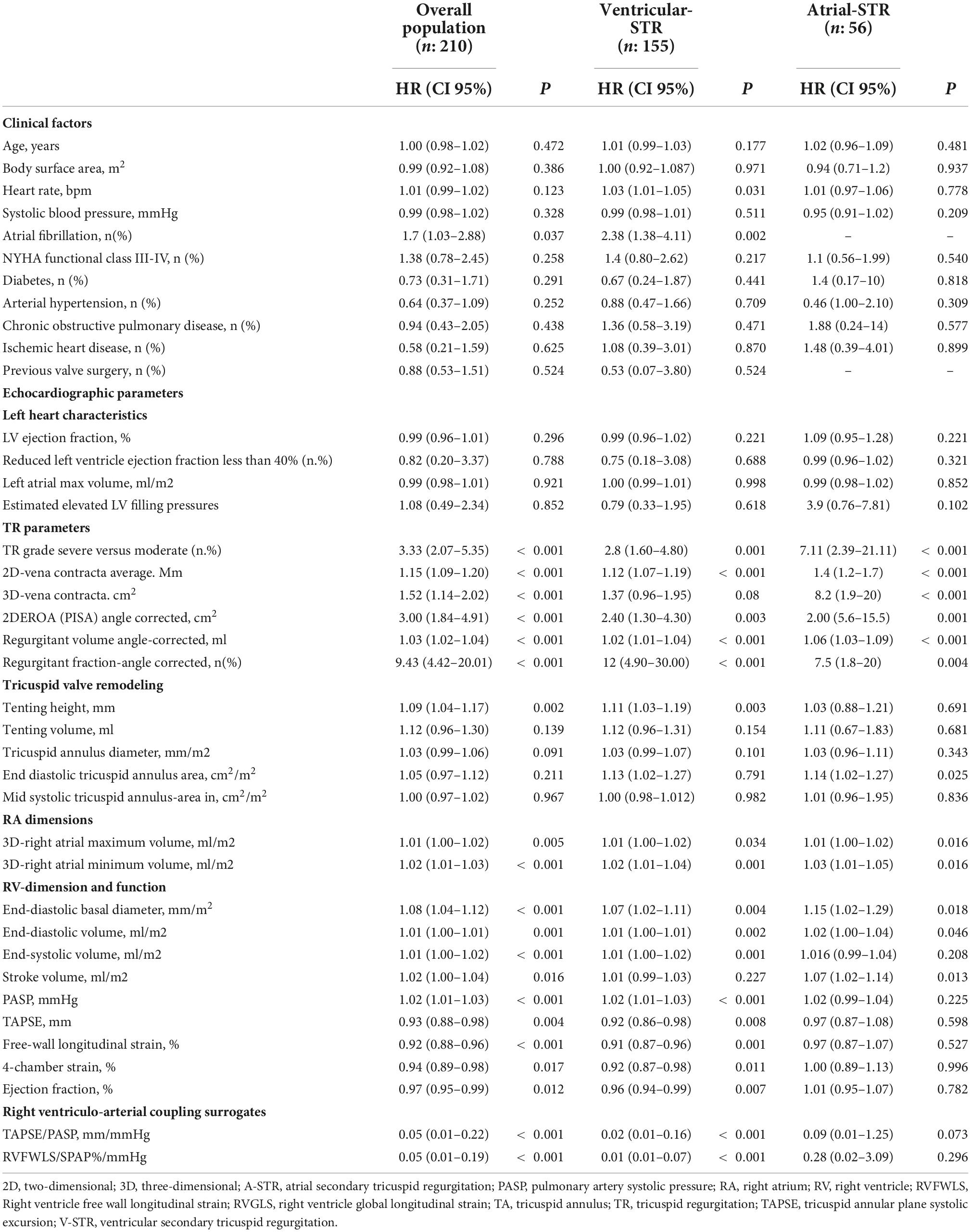
Table 3. Univariate Cox regression analysis of factors related to the combined endpoint in the whole cohort and in the 2 distinct phenotypes of STR.
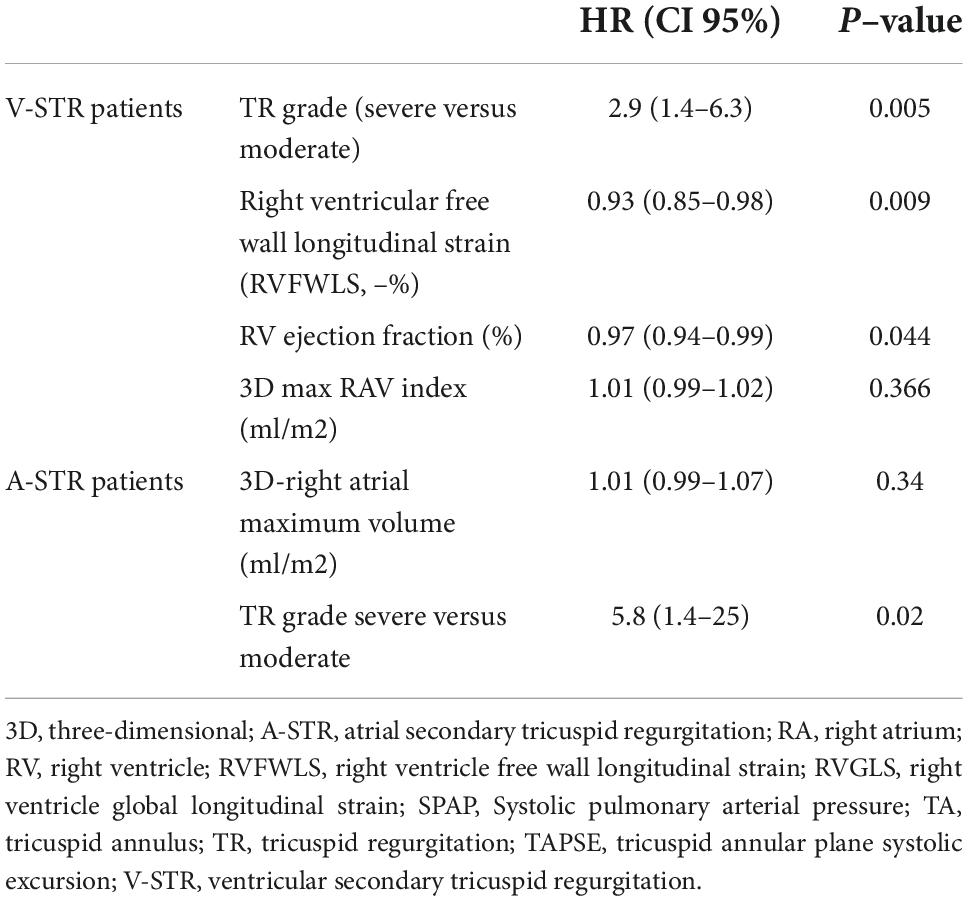
Table 4. Multivariable Cox regression to identify the variables associated with the combined endpoint in patients with atrial and ventricular secondary tricuspid regurgitation.
Discussion
The present is the first study aiming to compare the outcome and its associated factors among patients with moderate or severe STR, classified according to recent guidelines in A-STR and V-STR. Our results show that (i) patients with A-STR are older and less symptomatic than V-STR patients, with fewer leaflet tethering, larger TA size, and more preserved RV function and RV-PA coupling. (ii) A-STR patients have a significantly lower incidence of death and hospitalization for HF at follow-up. (iii) While STR severity is the only parameter independently associated with prognosis in A-STR, RV function (in addition to STR severity) had prognostic relevance in V-STR.
Difference in outcomes and right chamber remodeling of atrial secondary tricuspid regurgitation and ventricular secondary tricuspid regurgitation
In the last years, the growing interest in interventional treatment of TR has raised the need of having a precise characterization of patients with significant STR, and a nomenclature that could classify patients with STR according to the main pathogenetic mechanism. Only recently, the clinical and echocardiographic parameters to be used to identify A-STR have been reported (12, 13, 31). Accordingly, A-STR has been recognized as a distinct pathophysiological entity, and its mechanisms have begun to be studied (5–7, 32, 33). Also, A-STR is not a benign condition. Patients with isolated or A-STR are frequently hospitalized for HF and experience excess mortality. Elevated right atrial pressure and renal dysfunction are associated with mortality. This poor outcome may have implications for timing of intervention (34). Abe et al. reported that, in AF patients with preserved LVEF, the combination of mitral and STR was associated with a combined endpoint that include: cardiac death, admission due to heart failure, or mitral and/or tricuspid valve surgery (35). Both the mitral and the TR grade were independently related to the combined endpoint, irrespectively from the other echocardiographic parameters (35).
Another study confirmed the independent role of STR in long-standing AF patients for predicting HF hospitalization and death for any cause (36). Finally, a recent study based on a clustering analysis (and no a priori assumption) identified three distinct phenotypes (clusters) of STR based on RV and RA volumes and function: cluster 1 included patients with better right ventricular, left ventricular, and right atrial function; cluster 2 with reduced RV and RA strain despite similar sizes; cluster 3 patients with severely dilated heart chambers associated to RV and RA dysfunctions. These 3 phenotypes were associated with different outcomes and, under non-interventional management, the phenotype corresponding to preserved RV size and preserved RA and RV functions had less incidence of the combined endpoint (death and HF hospitalization) (17).
In our study, A-STR patients were identified using the criteria proposed in the last ACC/AHA guidelines (12). Using this definition, A-STR patients were found to be older than patients with V-STR but with less advanced functional impairment. Moreover, A-STR patients had a 2.7 lower risk of death for any cause and HF hospitalization compared to V-STR patients. Regarding the different echocardiographic remodeling observed in the 2 population, interesting results regarding RA, TA, and RV remodeling were found, in line with previous evidence (8, 20, 37).
Recently, a study from our group sought to analyze the TV geometry in a cohort of patients with any degree of TR compared with a control cohort and classified in A- and V-STR on the base of an echocardiographic definition (20). Contrary to our results, in that study, A-STR patients showed larger RAV min compared to V-STR. The fact that in our study the RA dilatation is similar between the 2 groups may depend on different definitions of A- and V-STR in the methods, being the RA dimension not a criterium in our study; secondly, our study included only patients with at least moderate STR (more severe STR). Therefore, the results of these 2 studies should be considered as a continuum in the spectrum of different grades of severity of STR, reaffirming the predominant role of RA and TA dilatation in determining the grade of TR in A-STR (20). The 3D-assessed TA area (both at end diastole and mid-systole) was larger in A-STR than in V-STR and, therefore, despite lower tenting height, the tenting volume was similar to V-STR patients. The assessment of TA size using 2DE linear dimensions was unable to detect this important difference about the remodeling of the TA between these two phenotypes of STR, in line with previous evidence (38). At the same way, also the RV-basal linear end-diastolic diameter was similar in the 2 groups of A-STR and V-STR but lower RV volumes were found in the A-STR. All these results are an important confirmation of the fact that RA, TA and RV size assessment by two-dimensional echocardiography (2DE) and linear methods, has important limitations in patients with STR and should be integrated with 3DE. Finally, regarding RV functional assessment, RVEF was not significantly different in the 2 groups; conversely RVFWLS was higher in patients with A-STR. These results reflect the stronger dependency of RVEF from volume overload of significant STR, as already demonstrated (16, 39, 40).
Echocardiographic factors related to outcomes in atrial secondary tricuspid regurgitation and ventricular secondary tricuspid regurgitation
The novelty of the present study was to identify the parameters associated with outcomes in A-STR and V-STR patients. In the A-STR cohort, the prognosis was independently associated only with the severity of STR rather than the size and the function of the RV and RA. The fact that none of the parameters of RV function was associated with the incidence of the combined endpoint agrees with the results of a previous study on patients with isolated TR (without mention regarding the AF link), in which RV dysfunction was not related to clinical outcomes, but the RA pressure and the venous congestion did (34). As a clinical perspective, the independent role of the severity of TR confirms that in patients with A-STR the reduction of TR, per se, should represent the main goal of the treatment.
Conversely, in patients with V-STR, RV function, in addition to STR severity, was associated with outcome. Recently, in a large population analysis, the importance of RV function and PASP on the prognosis of patients with STR has been explored (19). In this study, the higher the extent of the RV damage and the pulmonary pressure, the lower the relative impact of significant STR. In our study, STR severity and RV functional parameters were found to be independently associated with prognosis in the cohort of V-STR patients. These results further reflect the intrinsic pathogenetic relationship between STR and RV functional impairment, which are self-maintaining as if in a vicious cycle, and, again, highlight the importance of reducing STR grade to achieve disruption of this vicious cycle, even in patients with RV dysfunction (41–44).
The results of the present study should be considered in the “spectrum” of the recent evidence from the studies comparing the atrial and ventricular etiologies of secondary mitral regurgitation (SMR) (45, 46). Indeed, these data confirm that the two phenotypes of atrioventricular valve regurgitation are characterized by different clinical and echocardiographic characteristics, as well as different prognosis. As speculated in the setting of secondary mitral regurgitation, our results may have important implications for patients’ selection for transcatheter treatment of STR, underlying the relative importance and the heterogeneity of different remodeling parameters in prognostic stratification.
Study limitations
The limitations of the present study are inherent to its observational design, which allows only hypothesis making and no causal inference. In addition, the sample size was relatively small. However, we selected patients with complete 3D and good quality datasets to provide robust parameters to characterize A-ATR and V-STR patients fully. The relatively small size of the study population and the low rate of events are limitations that should be accounted for in our survival analysis. Medical records about medical treatment were somehow imprecise and incomplete and were not included in the present analysis and evaluated. Furthermore, although our findings align with current literature, we cannot exclude a selection bias affecting our results. Studies with a larger cohort of patients and a longer follow-up may therefore be needed to confirm our results.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the study was approved by the Istituto Auxologico. The patients/participants provided their written informed consent to participate in this study.
Author contributions
MG and FH contributed to conception and design of the study, organized the database, performed the statistical analysis, and wrote the first draft of the manuscript. LB and DM reviewed the results and the manuscript and added his special contribution as expert of field. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This research was funded by the Italian Ministry of Health (research project #RF-2021-12374122).
Conflict of interest
Author MG receives personal fees from Abbot Vascular. Authors DM and LB were members of the speaker bureau of GE Healthcare and Philips Medical Systems, and received research support from GE Healthcare, Philips Medical Systems, and EsaOte.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer DM and handling editor declared their shared affiliation.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Topilsky Y, Maltais S, Medina Inojosa J, Oguz D, Michelena H, Maalouf J, et al. Burden of tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc Imaging. (2019) 12:433–42. doi: 10.1016/j.jcmg.2018.06.014
2. Vieitez JM, Monteagudo JM, Mahia P, Perez L, Lopez T, Marco I, et al. New insights of tricuspid regurgitation: a large-scale prospective cohort study. Eur Heart J Cardiovasc Imaging. (2021) 22:196–202. doi: 10.1093/ehjci/jeaa205
3. Taramasso M, Vanermen H, Maisano F, Guidotti A, La Canna G, Alfieri O. The growing clinical importance of secondary tricuspid regurgitation. J Am Coll Cardiol. (2012) 59:703–10. doi: 10.1016/j.jacc.2011.09.069
4. Lancellotti P, Pibarot P, Chambers J, La Canna G, Pepi M, Dulgheru R, et al. Multi-modality imaging assessment of native valvular regurgitation: an EACVI and ESC council of valvular heart disease position paper. Eur Heart J Cardiovasc Imaging. (2022) 23:e171–232. doi: 10.1093/ehjci/jeab253
5. Utsunomiya H, Itabashi Y, Mihara H, Berdejo J, Kobayashi S, Siegel RJ, et al. Functional tricuspid regurgitation caused by chronic atrial fibrillation: a real-time 3-dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging. (2017) 10:e004897. doi: 10.1161/CIRCIMAGING.116.004897
6. Najib MQ, Vinales KL, Vittala SS, Challa S, Lee HR, Chaliki HP. Predictors for the development of severe tricuspid regurgitation with anatomically normal valve in patients with atrial fibrillation. Echocardiography. (2012) 29:140–6. doi: 10.1111/j.1540-8175.2011.01565.x
7. Park J-H, Shin S-H, Lee M-J, Lee M-D, Shim H-I, Yoon J, et al. Clinical and echocardiographic factors affecting tricuspid regurgitation severity in the patients with lone atrial fibrillation. J Cardiovasc Ultrasound. (2015) 23:136–42. doi: 10.4250/jcu.2015.23.3.136
8. Guta AC, Badano LP, Tomaselli M, Mihalcea D, Bartos D, Parati G, et al. The pathophysiological link between right atrial remodeling and functional tricuspid regurgitation in patients with atrial fibrillation: a three-dimensional echocardiography study. J Am Soc Echocardiogr. (2021) 34:585–94.e1. doi: 10.1016/j.echo.2021.01.004
9. Muraru D, Addetia K, Guta AC, Ochoa-Jimenez RC, Genovese D, Veronesi F, et al. Right atrial volume is a major determinant of tricuspid annulus area in functional tricuspid regurgitation: a three-dimensional echocardiographic study. Eur Heart J Cardiovasc Imaging. (2021) 22:660–9. doi: 10.1093/ehjci/jeaa286
10. Sanfilippo AJ, Abascal VM, Sheehan M, Oertel LB, Harrigan P, Hughes RA, et al. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation. (1990) 82:792–7. doi: 10.1161/01.cir.82.3.792
11. Florescu DR, Muraru D, Volpato V, Gavazzoni M, Caravita S, Tomaselli M, et al. Atrial functional tricuspid regurgitation as a distinct pathophysiological and clinical entity: no idiopathic tricuspid regurgitation anymore. J Clin Med. (2022) 11:382. doi: 10.3390/jcm11020382
12. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2021) 143:e72–227. doi: 10.1161/CIR.0000000000000923
13. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. (2021) 60:727–800. doi: 10.1093/ejcts/ezab389
14. Muraru D, Guta A-C, Ochoa-Jimenez RC, Bartos D, Aruta P, Mihaila S, et al. Functional regurgitation of atrioventricular valves and atrial fibrillation: an elusive pathophysiological link deserving further attention. J Am Soc Echocardiogr. (2020) 33:42–53. doi: 10.1016/j.echo.2019.08.016
15. Fortuni F, Butcher SC, Dietz MF, van der Bijl P, Prihadi EA, de Ferrari GM, et al. Right ventricular-pulmonary arterial coupling in secondary tricuspid regurgitation. Am J Cardiol. (2021) 148:138–45. doi: 10.1016/j.amjcard.2021.02.037
16. Prihadi EA, van der Bijl P, Dietz M, Abou R, Vollema EM, Marsan NA, et al. Prognostic implications of right ventricular free wall longitudinal strain in patients with significant functional tricuspid regurgitation. Circ Cardiovasc Imaging. (2019) 12:e008666. doi: 10.1161/CIRCIMAGING.118.008666
17. Vely M, L’official G, Galli E, Kosmala W, Guerin A, Chen E, et al. Functional tricuspid regurgitation: a clustering analysis and prognostic validation of three echocardiographic phenotypes in an external cohort. Int J Cardiol. (2022) 365:140–7. doi: 10.1016/j.ijcard.2022.07.019
18. Dietz MF, Prihadi EA, van der Bijl P, Goedemans L, Mertens BJA, Gursoy E, et al. Prognostic implications of right ventricular remodeling and function in patients with significant secondary tricuspid regurgitation. Circulation. (2019) 140:836–45. doi: 10.1161/CIRCULATIONAHA.119.039630
19. Itelman E, Vatury O, Kuperstein R, Ben-Zekry S, Hay I, Fefer P, et al. The association of severe tricuspid regurgitation with poor survival is modified by right ventricular pressure and function: insights from SHEBAHEART big data. J Am Soc Echocardiogr. (2022) 35:1028–36. doi: 10.1016/j.echo.2022.06.012
20. Florescu DR, Muraru D, Florescu C, Volpato V, Caravita S, Perger E, et al. Right heart chambers geometry and function in patients with the atrial and the ventricular phenotypes of functional tricuspid regurgitation. Eur Heart J Cardiovasc Imaging. (2021) 23:930–40. doi: 10.1093/ehjci/jeab211
21. Benfari G, Antoine C, Miller WL, Thapa P, Topilsky Y, Rossi A, et al. Excess mortality associated with functional tricuspid regurgitation complicating heart failure with reduced ejection fraction. Circulation. (2019) 140:196–206. doi: 10.1161/CIRCULATIONAHA.118.038946
22. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2015) 16:233–70. doi: 10.1093/ehjci/jev014
23. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the american society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr. (2017) 30:303–71. doi: 10.1016/j.echo.2017.01.007
24. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2016) 29:277–314. doi: 10.1016/j.echo.2016.01.011
25. Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging. (2012) 13:1–46. doi: 10.1093/ehjci/jer316
26. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. (2018) 19:591–600.
27. Badano LP, Muraru D, Parati G, Haugaa K, Voigt J-U. How to do right ventricular strain. Eur Heart J Cardiovasc Imaging. (2020) 21:825–7. doi: 10.1093/ehjci/jeaa126
28. Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, et al. European association of echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr. (2010) 11:307–32. doi: 10.1093/ejechocard/jeq031
29. Tomaselli M, Badano LP, Menè R, Gavazzoni M, Heilbron F, Radu N, et al. Impact of correcting the 2D PISA method on the quantification of functional tricuspid regurgitation severity. Eur Heart J Cardiovasc Imaging. (2022) 23:1459–70. doi: 10.1093/ehjci/jeac104
30. Muraru D, Hahn RT, Soliman OI, Faletra FF, Basso C, Badano LP. 3-Dimensional echocardiography in imaging the tricuspid valve. JACC Cardiovasc Imaging. (2019) 12:500–15. doi: 10.1016/j.jcmg.2018.10.035
31. Praz F, Muraru D, Kreidel F, Lurz P, Hahn RT, Delgado V, et al. Transcatheter treatment for tricuspid valve disease. Eurointervention. (2021) 17:791–808. doi: 10.4244/EIJ-D-21-00695
32. Utsunomiya H, Harada Y, Susawa H, Ueda Y, Izumi K, Itakura K, et al. Tricuspid valve geometry and right heart remodelling: insights into the mechanism of atrial functional tricuspid regurgitation. Eur Heart J Cardiovasc Imaging. (2020) 21:1068–78. doi: 10.1093/ehjci/jeaa194
33. Ortiz-Leon XA, Posada-Martinez EL, Trejo-Paredes MC, Ivey-Miranda JB, Pereira J, Crandall I, et al. Understanding tricuspid valve remodelling in atrial fibrillation using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging. (2020) 21:747–55. doi: 10.1093/ehjci/jeaa058
34. Fender EA, Petrescu I, Ionescu F, Zack CJ, Pislaru SV, Nkomo VT, et al. Prognostic importance and predictors of survival in isolated tricuspid regurgitation: a growing problem. Mayo Clin Proc. (2019) 94:2032–9. doi: 10.1016/j.mayocp.2019.04.036
35. Abe Y, Akamatsu K, Ito K, Matsumura Y, Shimeno K, Naruko T, et al. Prevalence and prognostic significance of functional mitral and tricuspid regurgitation despite preserved left ventricular ejection fraction in atrial fibrillation patients. Circ J. (2018) 82:1451–8. doi: 10.1253/circj.CJ-17-1334
36. Prapan N, Ratanasit N, Karaketklang K. Significant functional tricuspid regurgitation portends poor outcomes in patients with atrial fibrillation and preserved left ventricular ejection fraction. BMC Cardiovasc Disord. (2020) 20:433. doi: 10.1186/s12872-020-01716-6
37. Topilsky Y, Khanna A, Le Tourneau T, Park S, Michelena H, Suri R, et al. Clinical context and mechanism of functional tricuspid regurgitation in patients with and without pulmonary hypertension. Circ Cardiovasc Imaging. (2012) 5:314–23. doi: 10.1161/CIRCIMAGING.111.967919
38. Volpato V, Lang RM, Yamat M, Veronesi F, Weinert L, Tamborini G, et al. Echocardiographic assessment of the tricuspid annulus: the effects of the third dimension and measurement methodology. J Am Soc Echocardiogr. (2019) 32:238–47. doi: 10.1016/j.echo.2018.09.008
39. Trip P, Kind T, van de Veerdonk MC, Marcus JT, de Man FS, Westerhof N, et al. Accurate assessment of load-independent right ventricular systolic function in patients with pulmonary hypertension. J Heart Lung Transplant. (2013) 32:50–5. doi: 10.1016/j.healun.2012.09.022
40. Vanderpool RR, Rischard F, Naeije R, Hunter K, Simon MA. Simple functional imaging of the right ventricle in pulmonary hypertension: can right ventricular ejection fraction be improved? Int J Cardiol. (2016) 223:93–4. doi: 10.1016/j.ijcard.2016.08.138
41. Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. (2008) 117:1717–31. doi: 10.1161/CIRCULATIONAHA.107.653584
42. Mangieri A, Montalto C, Pagnesi M, Jabbour RJ, Rodés-Cabau J, Moat N, et al. Mechanism and implications of the tricuspid regurgitation. Circ Cardiovasc Interv. (2017) 10:e005043. doi: 10.1161/CIRCINTERVENTIONS.117.005043
43. Kim H-K, Kim Y-J, Park J-S, Kim KH, Kim K-B, Ahn H, et al. Determinants of the severity of functional tricuspid regurgitation. Am J Cardiol. (2006) 98:236–42. doi: 10.1016/j.amjcard.2006.01.082
44. di Mauro M, Bezante GP, di Baldassarre A, Clemente D, Cardinali A, Acitelli A, et al. Functional tricuspid regurgitation: an underestimated issue. Int J Cardiol. (2013) 168:707–15. doi: 10.1016/j.ijcard.2013.04.043
45. Okamoto C, Okada A, Nishimura K, Moriuchi K, Amano M, Takahama H, et al. Prognostic comparison of atrial and ventricular functional mitral regurgitation. Open Heart. (2021) 8:e001574. doi: 10.1136/openhrt-2021-001574
Keywords: atrial secondary tricuspid regurgitation, atrial fibrillation, heart failure hospitalizations, secondary tricuspid regurgitation, prognosis
Citation: Gavazzoni M, Heilbron F, Badano LP, Radu N, Cascella A, Tomaselli M, Perelli F, Caravita S, Baratto C, Parati G and Muraru D (2022) The atrial secondary tricuspid regurgitation is associated to more favorable outcome than the ventricular phenotype. Front. Cardiovasc. Med. 9:1022755. doi: 10.3389/fcvm.2022.1022755
Received: 18 August 2022; Accepted: 15 November 2022;
Published: 29 November 2022.
Edited by:
Francesco Ancona, Servizio di Imaging Cardiovascolare, Ospedale San Raffaele (IRCCS), ItalyReviewed by:
Marcel Weber, University Hospital Bonn, GermanyMingxing Xie, Huazhong University of Science and Technology, China
Davide Margonato, San Raffaele Hospital (IRCCS), Italy
Copyright © 2022 Gavazzoni, Heilbron, Badano, Radu, Cascella, Tomaselli, Perelli, Caravita, Baratto, Parati and Muraru. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luigi P. Badano, luigi.badano@unimib.it
 Mara Gavazzoni
Mara Gavazzoni Francesca Heilbron
Francesca Heilbron Luigi P. Badano
Luigi P. Badano Noela Radu2,3
Noela Radu2,3  Andrea Cascella
Andrea Cascella Francesco Perelli
Francesco Perelli Sergio Caravita
Sergio Caravita Gianfranco Parati
Gianfranco Parati Denisa Muraru
Denisa Muraru